tales to tell.
March 27, 2009
New element found!
NEW ELEMENT IN THE PERIODIC TABLE!
Element : Women
Symbol : Wo+
Atomic mass : Accepted as 53.6 Kg; isotopes may vary from 40-200kg.
Atomic number: unknown. That's why men have no idea how to handle them.
Occurrence : Copious quantities in all urban areas.
*PHYSICAL PROPERTIES*
1. Boils at room temperature.
2. Freezes without any known reason.
3. Melts if given special treatment.
4. Bitter, if incorrectly used.
5. Sweet as Honey if given a proper treatment.
*CHEMICAL PROPERTIES*
1. Have great affinity for Gold, Silver and a range of precious stones and absorbs great quantities of expensive substances.
2. May explode spontaneously without prior warning and for no known reason.
3. Insoluble in liquids, but activity greatly increases by that.
4. Most powerful money reducing agent known to man.
*COMMON USES*
1. Highly ornamental, especially in sports cars.
2. Can be great aid to relaxation.
*TESTS*
1. Pure specimen turns rosy pink when happy.
2. Turns green when placed behind a better specimen.
*POTENTIAL HAZARD*
Illegal to possess more than one, although several can be maintained at different locations as long as specimens do not come in direct contact with each other.
*!! WARNING !!*
PROLONGED EXPOSURE TO THIS ELEMENT CAN CAUSE SEVERE FINANCIAL HEMORRHAGING AND MENTAL DISTRESS. BE CAUTIOUS. THE ABOVE PROPERTIES ARE SHOWN BY ALL THE WOMEN OF THE WORLD.
Hahas. :)
by ツバサ at 8:52:00 PM
_________________________________________________________
This has nothing whatsoever to do with Chemistry, but I felt obliged to mention this:
Yesterday, 5.45 pm.
The platform at Bukit Batok station was quite packed with people. The sky was quite cloudy with occasional flashes of lightning above the roof of West Mall. I paced impatiently on the platform, waiting for the train to come.
Finally, about five minutes later, it has arrived. I squeezed aboard the train, and began my journey home.
......
Bukit Gombak.
The train door opened, and the passengers hustled to alight or board the train.
And now, here was where it all began.
A pregnant lady board the train and moved towards me. I stepped aside for her to grap the handgrip and the train began to move. I glanced at the lady from the corner of my eye. She was gripping the handgrip so tightly that her knuckles were white as sheet. She seemed to be in some difficulty keeping herself balanced as the train ride was somehow bumpy.
......
Choa Chu Kang Interchange.
And there goes the bustling passengers. For a moment it was cleared of standing passengers, so the lady moved towards the seats. There were two men seated in front of her. One looked like a Chinese foreign worker, while the other was an Indian man.
Sadly, both of them ignored the lady and did not gave up their seats to her. I walked towards the two men and said, "Excuse me sirs, can you please allow this lady to take your seat? She's pregnant, you know."
The foreign worker turned his head to one side and closed his eyes and the Indian man took out a newspaper and buried his head into it.
The lady gave me a sad smile to thank for my effort and continued to struggle until she alighted at Kranji.
- - -
More non-Chemistry stuff.
Cinquains I wrote for the Literature homework:
Behind
the fading moon
where night is dark and queer
a girl stood crying, then looks up
and jumped.
* * *
Beyond
the dimension
a boy ran away from
his fear, his own self, but not his
destiny.
by ツバサ at 8:26:00 PM
_________________________________________________________
March 25, 2009
An update on my old career aspiration.
I mentioned in the career aspiration post that I am going to update on an interview with a friend of mine, who was a digital artist/graphic designer. So here it goes:
*-A Tea with Ocarina-sensei-*
(Note: T - me, O - my friend)
T: Why did you choose this career path?
O: I've been drawing a lot since young. I love doodling. So when I was about to choose my career path, I wanted to do something that (1) I was truly interested in; and (2) I was relatively good at. Since I probably would be stuck with the career I chose for all my life, it better be something I enjoy.
T: Yeah, I agree. It's very tiring to do something which you don't like, especially for life. But what inspires you to be a digital artist?
O: For being an "artist", there's not a certain person or an event that especially inspired me. As I said I loved drawing all along, so I'm...self-inspired? Haha. Maybe I should say my mother helped me the most to be who I am today, because she was always supportive and encouraged me to draw more, as well as always gave me comments to help me improve.
For being a "digital" artist, the thing that inspired me was Microsoft Word clipart. I was quite bad with traditional media such as oil color or charcoal, and I was a bit disheartened that maybe I couldn't become a fine artist. Then windows system came into normal families (before that it was DOS system which had almost no graphic at all), and for the first time in my life I realized that computer graphics looked just as good too. Oh yeah, there's no heart-moving stories to what actually inspires me. (Laughs.)
T: Okay, I see. So what are your thoughts and feelings for this career?
O: I love it, because it's something that I have passion for. Though sometimes after working on a piece of work for ten hours straight, I might swear that I would never never ever draw because it's so exhausting. But before I knew it, I was drawing again.
Being in art industry has this advantage: It's romantic, impressive, and makes people quickly admire you (haha). If I say "I do accounting", people won't say "Wow, I adore how you count the numbers!". But they'll always say "I wish to draw as nice as you". Seriously, this job is tiring. Long hours, high competition, may encounter unreasonable client, may have last-minute changes... But as long as you have the passion, everything's okay.
T: Whoa, it does sound tiring to me. Speaking of which, besides all the monotonous drawing and more drawing, did anything interesting ever happened when you took this job?
O: One interesting fact: I'm enlightened with how tight deadlines could be. When you think it's the tightest already, there's always a tighter one.
And basically everything that's said in "Murphy’s law - about designers". Artists/designers need to be able to 苦中作乐. (Translation: find joy in your boring work to make it seems interesting to you.)
T: Anyway, do you have any advice to for those who would like to choose this career path?
O: To those who're not sure if it's a good career to choose: Unless you're really really interested, don't jump onto the bandwagon. There're lots of digital artists nowadays, but it's hard to be a good one. In my poly days about one-third of my batch couldn't graduate. They either quitted, failed certain modules, or transferred to another course when they found that it was not as easy as they thought. This is a tiring path, seriously. Pictures are pleasant to look at, but not easy to draw. To be able to cope, you must practise in your free time. So you'd better be interested in it.To those who would choose this career: develop your own style. You can't go anywhere if you just follow someone else's style.
T: Hear that, digital-artist-to-be guys? To wrap this interview up, do you have any comments to make regarding your career?
O: Being good in art itself is not enough, because it's so hard to really stand out from the rest with one skill. Try to develop a second advantage of yours, say, storytelling. Thus, you may not be the best artist, and you may not be the best storyteller. But two skills combined, you now have more chances to be better than the other "digital artist + storytellers", because there'll be fewer of them.
T: Okay, people. Do you have what it takes to be a digital artist/graphic designer? And thanks to Ocarina-sensei who took some time off from her already overflowing schedule to help me complete this interview session.
- - -
PS Hey Jesslyn, thought maybe you would be interested in this simple interview format (between friends) to help you in your career.
by ツバサ at 11:58:00 PM
_________________________________________________________
Atomic Structure lessons.
I wasn't prepared for the first lesson, but I was able to follow Mr Tan quite well. The first lesson was pretty much about recalling the basic concepts from my O-levels. I know about isotopes, the sub-atomic particles (mass and charge of protons, neutrons and electrons) but when he came to the s, p, f, d, I got more attentive.
To be able to keep up with the lesson, I went back and did some research, but I was unable to understand anything.
So for the second lesson I was quite confused most of the time. Thanks to the new student Cindy, I somehow managed to keep up with the lesson. This reminds me about the post we did on our strength and weakness in Chemistry. I'm going to add in: strength - I'm a fast and quick learner. A sudden newfound talent.
Using the website (http://www.chemguide.co.uk/atoms/propsmenu.html#top) on the last page of the IT Activity worksheet that was given out today, I went to do more research on this topic...
(Half an hour later)
I think Cindy's explaination is easier to understand than all those research notes. Maybe I should 'interview' her on this topic to compile my notes. Haha.
- - -
Update (27/3/09).
I used my most trusted research website Wikipedia to collate my notes. And I finally understood the topic. Cheers.
Important terms related to this topic:
The orbital names (s, p, d, f) are derived from the characteristics of their spectroscopic lines: sharp, principal, diffuse and fundamental.
(Source - http://en.wikipedia.org/wiki/Atomic_orbital)
The shielding effect describes the decrease in attraction between an electron and the nucleus in any atom with more than one electron shell. It is also referred to as the screening effect or atomic shielding.
(Source - http://en.wikipedia.org/wiki/Screening_effect)
Elaboration:
Electrons that are nearer to the nucleus has higher energy compared to the electrons further away from the nucleus. This is because the electrons nearer to the nucleus intercept the energy to reach the electrons further away from the nucleus. Thus we can conclude that as the number of inner electrons increase, the shielding effect increases.
(Source - borrowed lecture notes collated by teacher from a student of Raffles Junior College)
Quantum chemistry is a branch of theoretical chemistry, which applies quantum mechanics and quantum field theory to address issues and problems in chemistry. The description of the electronic behavior of atoms and molecules as pertaining to their reactivity is one of the applications of quantum chemistry. Quantum chemistry lies on the border between chemistry and physics, and significant contributions have been made by scientists from both fields. It has a strong and active overlap with the field of atomic physics and molecular physics, as well as physical chemistry.
Quantum chemistry mathematically describes the fundamental behavior of matter at the molecular scale. It is, in principle, possible to describe all chemical systems using this theory. In practice, only the simplest chemical systems may realistically be investigated in purely quantum mechanical terms, and approximations must be made for most practical purposes (e.g., Hartree-Fock, post Hartree-Fock or Density functional theory, see computational chemistry for more details). Hence a detailed understanding of quantum mechanics is not necessary for most chemistry, as the important implications of the theory (principally the orbital approximation) can be understood and applied in simpler terms.
(Source - http://en.wikipedia.org/wiki/Quantum_chemistry)
Bohr model is a primitive model of the hydrogen atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics, and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems, the Bohr model is still commonly taught to introduce students to quantum mechanics, before moving on to the more accurate but more complex valence shell atom.
(Source - http://en.wikipedia.org/wiki/Bohr_Model)
Diagram on s, p, d, f:
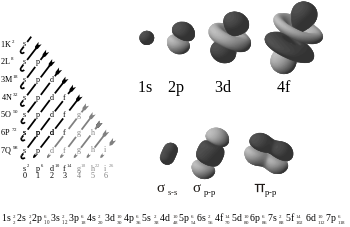
Note: this topic is very closely related to quantum physics.
by ツバサ at 11:40:00 PM
_________________________________________________________
March 24, 2009
End point vs equivalence point.
The equivalence point, or stoichiometric point, of a chemical reaction occurs during a chemical titration when the amount of titrant added is equivalent, or equal, to the amount of analyte present in the sample. In some cases there are multiple equivalence points which are multiples of the first equivalent point, such as in the titration of a diprotic acid. A graph of the titration curve exhibits an inflection point at the equivalence point. A striking fact about equivalence is that in a reaction the equivalence of the reactants as well as products is conserved.
The end point (similar, but not the same as the equivalence point) refers to the point at which the indicator changes color in a colorimetric titration.
To summarise and relate to our titration practicals, the equivalence point and the end point is actually the same, but the equivalence point is a much broader and general term used in chemical reactions which includes titration invloving pH indicator, redox, precipitation, conductivity of a solution, etc. However, the end point is only applicable to titration invloving pH indicator. Also, the end point in a titration involving pH indicator is an approximate value of its euivalence point.
pH indicator
This is a substance that changes colour in response to a chemical change. An acid-base indicator (e.g., phenolphthalein) changes colour depending on the pH. Redox indicators are also frequently used. A drop of indicator solution is added to the titration at the start; when the colour changes the endpoint has been reached, this is an approximation of the equivalence point.
[Source of research information - http://en.wikipedia.org/wiki/Endpoint_(chemistry)]
by ツバサ at 4:37:00 PM
_________________________________________________________
VA2.
I was unable to complete the practical because of time constraints, but it was hard to determine the end point for me. I kept having a dark shade of pink for two rounds.
And it seems that H2 Chemistry practical is not only testing our precision and lab skills, our understanding of theory concepts, but also our logic thinking. If you have no common sense at all, you shouldn't take Science- and Math-related subjects or courses in the first place.
When Ms Jee went through VA1, I finally see the obvious gap between the O and A Level demands. I have never done a VA of that intensity, and I was sweating madly doing that practical. But I'm getting back some of the basic skills that I've forgotten from my O's. And now I can do the practical faster and with more confidence.
All I can say is the demand of H2 Chemistry is so great that it's suffocating me. I have a bad feeling that I'm going to fall sick quite frequently this year.
- - -
Other subjects stuff.
Had Literature class today, and we were told to write a haiku (a form of Japanese poetry written with three lines and 5-7-5 syllabuses). Here's what I wrote:
The blue uniform
I stood by the oil lamp
And I took it off.
* * *
In the falling snow,
A forbidden wish has set
Another future.
by ツバサ at 4:00:00 PM
_________________________________________________________
March 22, 2009
My career aspiration.
Honestly, I have never ever thought that far. To me, my present is far more important. No present, no future. My current goal is to settle my A-Level stuff once and for all, in my three years in high school.
But since Ms Jee told us to do it for our own good (we must have a goal to aim for, you know), so here I am doing it and telling myself it's for my own good.
Actually, I have thought of being a novelist, a mangaka (artists who create mangas), a digital artist/graphic designer/web designer, a software writer...but now, my only aspiration is to become a doctor.
So why doctor and not any of my previous aspirations?
As I grow up and become more mature, I finally realised that it's time to be realistic. Looking at the real world out there, it seems that all my previous aspirations can be just a pastime, a hobby. When we're talking about career, we're talking about permanent jobs. Jobs that can feed you for your house, your meals. I know that it must be something I like, but I do like to be a doctor too (do you actually think that I chose that just for money?).
However, speaking of doctors, which kind do I want to be? I mean, there are so many different types of doctor-related jobs.
I did not actually think about it to that extent, so I'm not so sure myself. I went to do a bit of research, and my eyes are now in pain (there's so many...). I'm not going to elaborate about the requirements or criteria and whatsnot here (for all essential information, just go to wikipedia). There's no point copying and pasting for the sake of doing it. I wanted to interview my cousins (most of them are PhDs and Masters) but I am unable to get in touch with them...
Okay, I'm just being frank here. Still, to be able to study medicine in universities, I need to get straight-A's and there's an interview.
Haven't I said that dealing with my A-Level is more important than anything else?
I can dream, dream to become a doctor.
But my dream can never be fulfilled if I don't pay the price to grant it.
The price? The time I spent to do homework, to revise. The effort I have to put in to do my work. The attention I have to give at every single lectures and tutorials.
The wish I have made is high. And therefore the price I have to pay must be equal or more than my wish.
* * *
Nevermind, let's get on with my old aspirations. And I choose digital artist.
I have a friend who is a digital artist herself, and she draws for zbcomma, a Chinese newspaper for secondary school students. She is the one who taught me how to do CG using photoshop.
(She is currently unavailable for an interview because she's busy. Will update once I have interviewed her.)
You can go to her blog to see her art: echohall.spaces.live.com. I'm sorry if it's in Chinese, but just look at the pictures will do.
Here is one of the drafts I did using her tutorials when she was teaching me:

It's not finished yet, because I'm way too busy. Just look at my workload for the March holidays.
by ツバサ at 4:14:00 PM
_________________________________________________________
March 21, 2009
Les Strength vs Les Weakness.
So Ms Jee is interested in our strengths and weaknesses in Chemistry. Mine?
Weaknesses? Lots. Uncountable. But mainly Organic Chemistry and the memory work (I have a really bad memory - and I mean real bad). I am also bad at practical (I'm accident-prone). And I need time to get into the A-Level-Chemistry mood.
Strengths? Hey, I have not been studying Chemistry for like, four months? I have already lost all of my strengths for Chemistry, and I need time to get them back...so I say, none for the time being. Anyway, I was pretty strong in Chemistry in my secondary school days. Is that considered a strength?
Hang on, why didn't she ask me about the level of my love for Chemistry? =.=

There you go.
Haha.
by ツバサ at 11:59:00 AM
_________________________________________________________
March 2, 2009
Atoms, Moles and Stoichiometry: The Mathematics of Science and The Art of Science.
Wow. After I've read the article on the mathematics behind science, I finally realised the importance of SI units. By looking at the first table on page 1, I wondered how people of different nations would be able to trade with one another with different kinds of measurements. Surely it would be very confusing, but I am also curious what Singapore's system of units will be, seeing that the people are from different races?
Of course, I've already knew that there's only 7 base SI units from my physics lesson, but there's still an itchy question on my mind: how luminous intensity is measured? (Under candela.) And it's my first time I've ever heard of an unit called 'bar'.
Prefixes is something I'm supposed to know since I studied physics, though I would like to just add on one more line: pico, symbol p, factor 10^-12.
Regarding the example under the section on The Art of Science, I don't really like the working shown. Yes, I know I should throw away my what-I-was-being-taught-in-secondary-school mindset, but I felt that the method I've learnt in secondary school was a lot easier to understand, not to mention the working is clean and straight-forward. The method I was being taught in secondary school was to write the working in table form instead.
by ツバサ at 11:45:00 PM
_________________________________________________________
ツバサ © 2009. Hosted at Blogger.com. Blogskin ripped from Blogskins.com. All rights reserved.



